
How it works.
Continuous compliance.
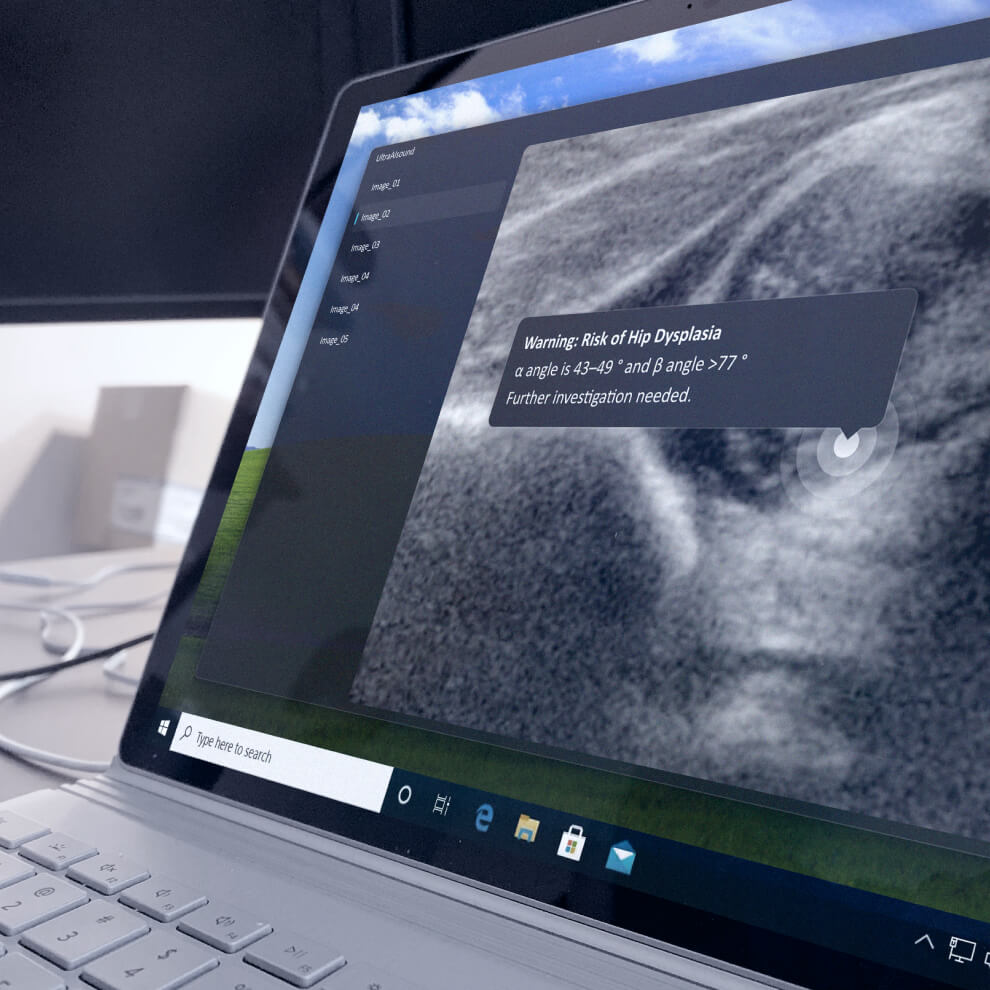
Scarlet is not a consultancy or an eQMS. Scarlet is a Notified Body with a structured interface specifically designed for Medical Software.
Our certification process is built to enable our customers to do frequent, compliant software releases, spanning regulatory jurisdictions.

Scarlet is trusted by the market and used by customers all around the world.
Our clients include everyone from early‑stage companies to established research hospitals, working on issues from skin cancer detection to remote patient monitoring, to chronic back pain management.

Our accreditations.
Scarlet is authorised and regulated to certify Software Medical Devices by the largest government agencies, including:
- ISO 17021-1:2015 certification by National accreditation agencies including UKAS and RVA;
- Ministries of Health, such as the Dutch Ministry of Health, Welfare and Sport, and the UK Medicines and Healthcare products Regulatory Agency.
- pan-government entities, such as the Health Department (DG-Santé) of the European Commission.
Commonly asked questions.
What standards can Scarlet certify against?
- Scarlet is accredited to issue ISO 13485:2016 certificates.
- Scarlet performs conformity assessment against Regulation 2017/745 (EU MDR) Annex IX, and against UK Medical Devices Regulations 2002 (SI 2002/618 as amended).
Which jurisdictions does Scarlet cover?
Scarlet covers the following jurisdictions:


Our approvals are also valid in countries that recognise EU MDR such as the EEA countries, Switzerland, and Turkey.
Does Scarlet certify hardware medical devices?
No, Scarlet only certifies Software and AI Medical Devices.
What’s Scarlet’s pricing structure?
Scarlet charges a flat monthly fee based on the scope of the overall software product you are certifying. This includes access to Scarlet’s interface and all certification services required to place and maintain your product on the market. There are no hidden fees.
Think Scarlet could help you? Get in touch.
Register to schedule a call or a demo, and get news and updates from Scarlet.




